Delving into which activity best demonstrates Ernest Rutherford’s creativity, this exploration immerses readers in a unique and compelling narrative. From his groundbreaking experiments to his innovative teaching methods, Rutherford’s creativity left an indelible mark on the field of physics and beyond.
His ability to think outside the box and come up with new ideas revolutionized our understanding of the atom and laid the foundation for modern nuclear physics.
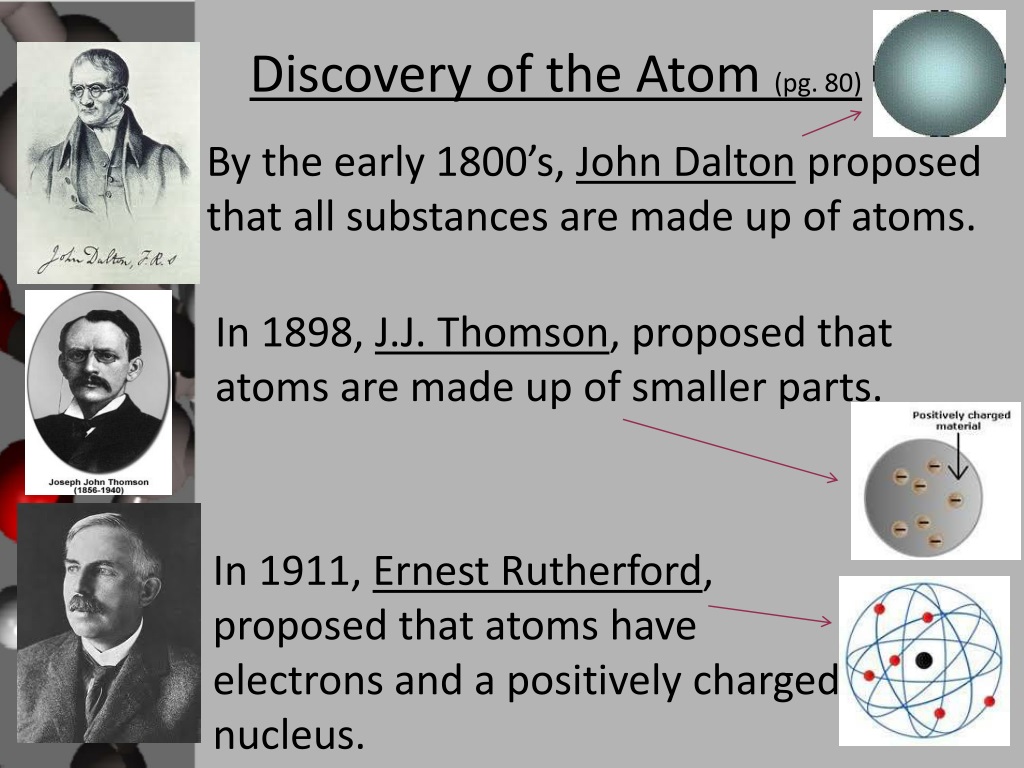
Rutherford’s most famous experiment, the gold foil experiment, was a turning point in the history of physics. By bombarding a thin sheet of gold foil with alpha particles, Rutherford discovered that most of the particles passed through the foil undeflected, while a small number were deflected at large angles.
This result led him to propose the nuclear model of the atom, which suggested that the atom has a small, dense nucleus surrounded by orbiting electrons. This model overturned the prevailing plum pudding model and laid the foundation for our modern understanding of atomic structure.
Gold Foil Experiment: Which Activity Best Demonstrates Ernest Rutherford’s Creativity
Ernest Rutherford’s Gold Foil Experiment was a groundbreaking experiment in physics that led to the discovery of the atomic nucleus. Conducted in 1911, the experiment involved firing a beam of alpha particles (helium nuclei) at a thin sheet of gold foil.
Rutherford expected most of the alpha particles to pass straight through the foil, with only a small number being deflected. However, the results of the experiment were unexpected. A significant number of alpha particles were deflected at large angles, some even bouncing back from the foil.
Significance of the Results
The results of the Gold Foil Experiment were significant because they contradicted the prevailing model of the atom at the time. The accepted model, proposed by J.J. Thomson, suggested that atoms were composed of a positively charged sphere with electrons embedded in it, like raisins in a cake.
Rutherford’s experiment showed that this model could not be correct. The large deflections of the alpha particles indicated that the positive charge in the atom was concentrated in a very small, dense nucleus. This nucleus was much smaller than the atom itself, and it contained most of the atom’s mass.
Creativity of Rutherford
The Gold Foil Experiment demonstrated Rutherford’s creativity in several ways. First, he was willing to challenge the accepted model of the atom, even though it was widely accepted by the scientific community.
Second, he designed an experiment that was simple and elegant, yet powerful enough to provide clear evidence for the existence of the atomic nucleus. Finally, he was able to interpret the results of his experiment in a way that led to a new understanding of the structure of the atom.
– Provide a timeline of Rutherford’s experiments leading to the nuclear model.

Ernest Rutherford conducted a series of experiments from 1899 to 1911 that culminated in the discovery of the nuclear model of the atom. Here is a timeline of his key experiments:
1899: Discovery of Alpha and Beta Particles
Rutherford and Frederick Soddy discovered alpha and beta particles while studying uranium radiation. Alpha particles were identified as helium nuclei, while beta particles were identified as electrons.
1908: Scattering of Alpha Particles from Gold Foil
Rutherford’s most famous experiment involved scattering alpha particles from a thin sheet of gold foil. The results of this experiment led to the discovery of the atomic nucleus.
1911: Nuclear Model of the Atom
Based on the results of his alpha particle scattering experiment, Rutherford proposed the nuclear model of the atom. This model describes the atom as having a small, dense nucleus surrounded by orbiting electrons.
Transmutation of Elements

Ernest Rutherford’s groundbreaking experiments on transmutation of elements revolutionized our understanding of the atom and paved the way for the development of nuclear physics. In this section, we will explore the significance of his discoveries, analyze how they demonstrate his creativity, and discuss the ethical implications and limitations of his work.
Rutherford’s Experiments
In 1919, Rutherford and his team bombarded nitrogen atoms with alpha particles (helium nuclei). Surprisingly, they observed the emission of protons, indicating that the nitrogen atoms had been transformed into oxygen atoms. This was the first time an element had been artificially transmuted into another.
Rutherford continued his experiments, bombarding various elements with alpha particles. He discovered that certain elements, such as aluminum and silicon, could also be transmuted into different elements. These experiments provided strong evidence for the existence of the atomic nucleus and the possibility of changing the composition of atoms.
Significance of Rutherford’s Discoveries
Rutherford’s experiments on transmutation had several important implications:
- They confirmed the existence of the atomic nucleus, which Rutherford had proposed in his earlier experiments on alpha scattering.
- They demonstrated that the atom was not an indivisible unit, as previously believed, but could be transformed into different elements.
- They opened up the possibility of using nuclear reactions to produce new elements and isotopes, with potential applications in medicine, energy production, and other fields.
Creativity and Challenges
Rutherford’s experiments on transmutation showcased his creativity and experimental ingenuity. He was not afraid to challenge established ideas and to explore new and uncharted territory.
However, these experiments were not without their challenges. Bombarding atoms with alpha particles was a dangerous and unpredictable process, and Rutherford and his team had to take great precautions to protect themselves from radiation exposure.
Ethical Implications
The potential of transmutation experiments to produce new elements and isotopes raised important ethical questions. Rutherford and other scientists recognized that these discoveries could be used for both beneficial and harmful purposes, such as the development of nuclear weapons.
As a result, scientists have established strict ethical guidelines for the use of nuclear technology, including the need for careful oversight and responsible decision-making.
Key Findings and Limitations
Rutherford’s experiments on transmutation led to several key findings:
- Elements can be artificially transmuted into other elements through nuclear reactions.
- The atomic nucleus is a small, dense region at the center of the atom that contains most of its mass.
- Nuclear reactions can release enormous amounts of energy, which can be harnessed for peaceful or destructive purposes.
However, Rutherford’s experiments also had limitations. He was unable to transmute all elements, and he did not fully understand the mechanisms involved in nuclear reactions.
Despite these limitations, Rutherford’s experiments laid the foundation for the development of nuclear physics and continue to inspire scientists today.
Areas for Future Research
Rutherford’s experiments opened up a vast field of research in nuclear physics. Ongoing and future research in this area includes:
- Developing new methods for transmuting elements
- Studying the properties of new elements and isotopes
- Exploring the potential applications of nuclear reactions in medicine, energy production, and other fields
Contributions to Nuclear Physics
Ernest Rutherford’s pioneering work in nuclear physics revolutionized our understanding of the atom. His groundbreaking experiments provided crucial insights into the structure and properties of atoms, laying the foundation for modern nuclear physics.
One of Rutherford’s most significant contributions was his discovery of the atomic nucleus. Through his famous Gold Foil Experiment, he demonstrated that most of the atom’s mass is concentrated in a tiny, dense core called the nucleus. This discovery shattered the prevailing plum pudding model of the atom, which envisioned electrons evenly distributed throughout the atom’s volume.
Rutherford also made important contributions to our understanding of radioactivity. He identified alpha and beta particles as distinct types of radiation emitted by radioactive elements. His experiments led to the development of the Rutherford-Soddy model of radioactive decay, which explained the transmutation of elements during radioactive processes.
Rutherford’s creativity and ingenuity were evident in his experimental approach. He devised innovative techniques, such as using thin gold foils and scintillation screens, to probe the structure of atoms. His willingness to challenge established theories and his relentless pursuit of knowledge pushed the boundaries of scientific understanding.
Scattering Experiments
Rutherford’s Gold Foil Experiment was a pivotal moment in nuclear physics. By bombarding a thin gold foil with alpha particles, he observed that most particles passed through the foil undeflected, while a small number were deflected at large angles. This unexpected result led him to propose the nuclear model of the atom, with a dense, positively charged nucleus surrounded by a cloud of electrons.
Nuclear Transmutation, Which activity best demonstrates ernest rutherford’s creativity
Rutherford’s experiments on the bombardment of nitrogen atoms with alpha particles led to the discovery of nuclear transmutation. He observed the formation of oxygen atoms and hydrogen nuclei, demonstrating that one element could be transformed into another through nuclear reactions.
This discovery laid the foundation for nuclear chemistry and paved the way for the development of nuclear power and nuclear weapons.
Rutherford’s Model of the Atom
Rutherford’s nuclear model of the atom revolutionized our understanding of atomic structure. He proposed that atoms consist of a tiny, dense nucleus surrounded by a cloud of electrons. The nucleus contains most of the atom’s mass and is positively charged, while the electrons are negatively charged and orbit the nucleus.
This model provided a framework for understanding the chemical and physical properties of elements and became the basis for modern atomic theory.
Experimental Techniques

Rutherford’s experimental techniques were groundbreaking in the field of physics, providing crucial evidence for the nuclear model of the atom. His innovative use of alpha particles and gold foil led to key discoveries that revolutionized our understanding of atomic structure.
Rutherford’s experimental approach was characterized by creativity and ingenuity. He overcame experimental challenges by devising novel techniques and interpreting results with great insight. His work set the stage for further advancements in nuclear physics and laid the foundation for our modern understanding of the atom.
Alpha Particle Scattering Experiment
In his famous gold foil experiment, Rutherford bombarded a thin sheet of gold foil with alpha particles. Alpha particles are positively charged helium nuclei with high energy. By observing the scattering patterns of the alpha particles, Rutherford deduced the existence of a small, dense nucleus at the center of the atom.
Most alpha particles passed through the foil undeflected, indicating that most of the atom’s volume was empty space. However, a small number of alpha particles were deflected at large angles, suggesting they had encountered a strong repulsive force. Rutherford concluded that this force must be due to a positively charged nucleus concentrated in a tiny region within the atom.
Rutherford’s experiment provided strong evidence for the nuclear model of the atom, where the mass and positive charge of the atom are concentrated in a central nucleus, surrounded by orbiting electrons.
Timeline of Key Experimental Breakthroughs
- 1908: Alpha particle scattering experiment
- 1911: Discovery of the atomic nucleus
- 1919: First artificial nuclear transmutation
Table of Experimental Techniques
| Technique | Purpose | Key Findings |
|---|---|---|
| Alpha particle scattering | To investigate the structure of the atom | Discovery of the atomic nucleus |
| Artificial nuclear transmutation | To transform one element into another | First evidence of nuclear reactions |
Broader Implications
Rutherford’s experimental approach had far-reaching implications for scientific inquiry and the nature of scientific progress. His emphasis on experimental evidence and his willingness to challenge established theories set an example for future generations of scientists.
Rutherford’s work also highlighted the importance of collaboration and the exchange of ideas. He worked closely with other physicists, including Niels Bohr and James Chadwick, and their collective efforts led to major breakthroughs in our understanding of the atom.
Collaboration and Mentorship
Ernest Rutherford’s scientific journey was greatly influenced by his collaborative spirit and mentorship of young scientists. His ability to foster a collaborative environment and inspire future generations of physicists showcased his creativity beyond his groundbreaking experiments.
Rutherford’s mentorship extended to several notable scientists, including Niels Bohr, James Chadwick, and Otto Hahn. He provided guidance and support, encouraging them to pursue their own research interests and challenge established norms. His mentorship style fostered a culture of intellectual curiosity and critical thinking, shaping the scientific landscape of the 20th century.
Influence on Other Scientists
- Niels Bohr:Rutherford’s collaboration with Bohr led to the development of the Bohr model of the atom, a groundbreaking theory that revolutionized our understanding of atomic structure.
- James Chadwick:Under Rutherford’s guidance, Chadwick discovered the neutron, a fundamental particle that plays a crucial role in nuclear reactions.
- Otto Hahn:Hahn’s work on nuclear fission, which ultimately led to the development of nuclear energy, was influenced by Rutherford’s mentorship and encouragement.
Rutherford’s collaborative spirit extended beyond his direct mentorship. He actively engaged with the scientific community, fostering collaborations and exchanging ideas. This open and inclusive approach created a fertile environment for scientific progress, where diverse perspectives could converge and lead to groundbreaking discoveries.
Scientific Communication
Ernest Rutherford possessed an extraordinary ability to communicate complex scientific concepts with clarity and precision. His writing and lectures were instrumental in shaping the field of physics and inspiring a new generation of scientists.
Writing
Rutherford’s writing was characterized by its clarity, conciseness, and logical organization. He had a gift for explaining complex ideas in a way that was accessible to both scientists and laypeople. His most famous work, “Radioactivity,” published in 1904, became a standard textbook for the study of radioactivity.
Lectures
Rutherford was also a gifted lecturer. His lectures were known for their engaging style, humor, and ability to convey complex concepts in a memorable way. He often used simple experiments and demonstrations to illustrate his points, making his lectures both informative and entertaining.
Creativity
Rutherford’s communication skills were a testament to his creativity. He had a knack for finding new and innovative ways to explain complex scientific concepts. His ability to communicate his ideas effectively was essential to the success of his research and the advancement of physics.
Theoretical and Experimental Approach
Ernest Rutherford’s unique combination of theoretical and experimental work was a cornerstone of his success in physics. He believed that theoretical understanding should guide experimentation, and that experimental results should inform theory. This approach allowed him to make significant discoveries in the field of atomic physics, including the discovery of the atomic nucleus.
Theoretical Underpinnings
Rutherford’s theoretical work was based on the classical laws of physics, particularly electromagnetism. He used these laws to develop models of the atom that could explain the experimental results he observed. For example, he proposed the “plum pudding” model of the atom, in which the electrons were embedded in a positively charged sphere.
Experimental Breakthroughs
Rutherford’s experimental work was equally groundbreaking. He developed new techniques for studying the atom, such as the gold foil experiment. In this experiment, he bombarded a thin sheet of gold foil with alpha particles (helium nuclei). Most of the alpha particles passed through the foil without being deflected, but a small number were deflected at large angles.
This result led Rutherford to conclude that the atom must have a small, dense nucleus at its center.
Balanced Approach
Rutherford’s balanced approach to physics was essential to his success. His theoretical work provided him with the framework for understanding his experimental results, and his experimental work provided him with the data to support his theories. This combination of theory and experiment allowed him to make significant advances in our understanding of the atom.
Impact on Physics
Rutherford’s work had a profound impact on the field of physics. His discovery of the atomic nucleus led to the development of the nuclear model of the atom, which is still the accepted model today. His work also laid the foundation for the development of nuclear physics, which has led to the development of nuclear power and nuclear weapons.
Implications for Future Research
Rutherford’s approach to physics continues to be a model for scientists today. His emphasis on the importance of both theory and experiment has led to the development of many new scientific discoveries. His work is a reminder that the best science is done when theory and experiment are combined.
Problem Solving
Ernest Rutherford’s approach to problem-solving was characterized by his creativity and ingenuity. He was not afraid to challenge conventional wisdom and was always willing to experiment with new ideas.
One of the most famous examples of Rutherford’s problem-solving skills is his work on the structure of the atom. At the time, it was believed that atoms were indivisible and that the electrons orbited the nucleus in a circular path.
However, Rutherford’s experiments showed that this model was incorrect.
Rutherford’s Gold Foil Experiment
In his famous gold foil experiment, Rutherford fired a beam of alpha particles at a thin sheet of gold foil. He expected the alpha particles to pass straight through the foil, but instead, he found that some of them were deflected at large angles.
This led him to conclude that the atom must have a small, dense nucleus surrounded by a cloud of electrons.
Rutherford’s problem-solving skills were not limited to his work on the atom. He also made important contributions to the fields of nuclear physics and radioactivity. His work helped to lay the foundation for our modern understanding of the atom and its structure.
Ernest Rutherford’s creativity is best exemplified by his famous gold foil experiment. However, for those seeking to foster creativity in children, the creative world montessori approach offers a comprehensive framework. This method emphasizes hands-on exploration, self-directed learning, and a supportive environment, nurturing the creativity that lies within every child, just like Rutherford’s groundbreaking experiment.
Inspiration and Imagination
Ernest Rutherford’s groundbreaking discoveries were fueled by a combination of inspiration and imagination. His mentors, peers, and the scientific environment of his time provided a fertile ground for his ideas to flourish.
Rutherford’s imagination played a crucial role in his scientific endeavors. He often used thought experiments and visualization to advance his understanding. For example, his famous Gold Foil Experiment was inspired by a thought experiment in which he imagined alpha particles scattering off a nucleus.
Sources of Inspiration
Rutherford was inspired by a wide range of sources, including:
- His mentors, such as J.J. Thomson and Heinrich Hertz
- His peers, such as Niels Bohr and Marie Curie
- The scientific environment of his time, which was characterized by rapid advances in physics
Role of Imagination
Rutherford’s imagination manifested itself in his scientific work in a number of ways:
- He used thought experiments to test his ideas and develop new hypotheses.
- He visualized the atomic structure of elements, which helped him to understand their behavior.
- He was able to see patterns in data that others missed, which led to new discoveries.
Influence on Scientific Endeavors
Inspiration and imagination played a major role in Rutherford’s scientific endeavors:
- They influenced his research questions, leading him to explore new and uncharted territory.
- They helped him to develop innovative experimental designs, which allowed him to test his ideas in new and creative ways.
- They enabled him to interpret his results in a way that led to new discoveries.
Essay
The relationship between inspiration and imagination in Rutherford’s scientific work was a complex and dynamic one. Inspiration provided the spark that ignited his curiosity, while imagination provided the fuel that drove his discoveries. Without both of these qualities, it is unlikely that Rutherford would have made the groundbreaking contributions to science that he did.
Legacy and Impact
Ernest Rutherford’s pioneering work in nuclear physics left an enduring legacy on the field. His discovery of the atomic nucleus revolutionized our understanding of the atom and laid the foundation for the development of nuclear energy and medical applications.
Scientific Discoveries
Rutherford’s key discoveries include:
- Discovery of the atomic nucleus:His Gold Foil Experiment demonstrated the existence of a tiny, dense nucleus at the center of the atom, contradicting the prevailing “plum pudding” model.
- Understanding of radioactivity:He identified alpha and beta particles as distinct forms of radiation, and developed the theory of radioactive decay.
- Transmutation of elements:He was the first to artificially transmute one element into another, bombarding nitrogen with alpha particles to create oxygen.
Impact on Physics
Rutherford’s discoveries had a profound impact on physics:
- Development of the nuclear model of the atom:His work led to the development of the Rutherford model of the atom, which depicted the atom as a tiny nucleus surrounded by orbiting electrons.
- Understanding of radioactivity:His theory of radioactive decay provided a framework for understanding the behavior of radioactive elements and their applications in fields such as medicine and dating techniques.
- Foundation for nuclear energy and technology:His work on nuclear transmutation laid the groundwork for the development of nuclear reactors and nuclear weapons.
Legacy
Rutherford’s legacy is one of creativity, innovation, and groundbreaking discoveries. His willingness to challenge established theories and his experimental approach have inspired generations of physicists.
“Rutherford was one of the greatest experimental physicists of all time. His work on the atomic nucleus revolutionized our understanding of the atom and laid the foundation for modern physics.”- Stephen Hawking
Influence on Modern Physics and Technology
Rutherford’s work continues to influence modern physics and technology:
- Nuclear energy:His discoveries led to the development of nuclear reactors, which generate electricity and power submarines and spacecraft.
- Medical applications:Radioactive isotopes discovered by Rutherford are used in medical imaging and cancer treatment.
- Particle accelerators:His work on the transmutation of elements laid the groundwork for the development of particle accelerators, which are used in high-energy physics research.
Awards and Recognition
Ernest Rutherford’s groundbreaking contributions to nuclear physics earned him numerous prestigious awards and recognitions.
In 1908, he was awarded the Nobel Prize in Chemistry for his investigations into the disintegration of the elements, and the chemistry of radioactive substances.
In 1914, he was knighted for his scientific achievements. He was also awarded the Copley Medal of the Royal Society in 1922, the Faraday Medal of the Institution of Electrical Engineers in 1924, and the Franklin Medal of the Franklin Institute in 1925.
These awards and recognitions not only celebrated Rutherford’s scientific brilliance but also highlighted the transformative impact of his creativity on the field of physics.
Nobel Prize in Chemistry
The Nobel Prize in Chemistry, awarded to Rutherford in 1908, was a testament to his groundbreaking work on radioactivity and the disintegration of elements.
His experiments demonstrated that radioactive elements emitted three types of radiation, which he named alpha, beta, and gamma rays. He also showed that alpha particles were helium nuclei, a discovery that laid the foundation for the development of nuclear physics.
Knighthood
In 1914, Rutherford was knighted by King George V for his outstanding contributions to science.
This honor recognized not only his scientific achievements but also his role as a leader in the scientific community. Rutherford was a gifted communicator and teacher, and he inspired a generation of young scientists to pursue careers in physics.
Copley Medal
The Copley Medal, awarded by the Royal Society in 1922, is one of the most prestigious awards in science.
It is given to scientists who have made outstanding contributions to the advancement of knowledge. Rutherford’s receipt of this award was a recognition of his groundbreaking work on the structure of the atom and the development of nuclear physics.
Faraday Medal
The Faraday Medal, awarded by the Institution of Electrical Engineers in 1924, is given to scientists who have made significant contributions to the field of electrical engineering.
Rutherford’s receipt of this award was a recognition of his work on the development of the electron microscope and his contributions to the understanding of the electrical properties of materials.
Franklin Medal
The Franklin Medal, awarded by the Franklin Institute in 1925, is given to scientists who have made outstanding contributions to the advancement of science.
Rutherford’s receipt of this award was a recognition of his groundbreaking work on the structure of the atom and the development of nuclear physics.
Contributions to Science Education
Ernest Rutherford’s contributions to science education were profound and far-reaching. He played a pivotal role in developing the nuclear model of the atom and promoting scientific literacy through his lectures and writings.
Nuclear Model of the Atom
Rutherford’s groundbreaking Gold Foil Experiment led to the development of the nuclear model of the atom. This model revolutionized our understanding of the atom’s structure, showing that it consists of a tiny, dense nucleus surrounded by orbiting electrons.
Promotion of Scientific Literacy
Rutherford was a passionate advocate for scientific literacy. He gave numerous public lectures and wrote extensively, making complex scientific concepts accessible to a wider audience. His writings and lectures helped foster scientific curiosity and understanding among the general public.
Innovative Teaching Methods
Rutherford was an innovative teacher who introduced laboratory-based experiments and encouraged student participation in research. He believed that hands-on learning and critical thinking were essential for effective science education.
Influence on Contemporary Teaching
Rutherford’s ideas on scientific inquiry and student-centered learning continue to influence modern science education practices. His emphasis on inquiry-based approaches and student engagement has shaped the way science is taught and learned today.
Collaboration with Marie Curie
Ernest Rutherford and Marie Curie, two pioneering scientists of their time, collaborated on groundbreaking research that significantly advanced our understanding of radioactivity and nuclear physics.
In 1903, Rutherford and Curie began working together to study the properties of radioactive elements. Rutherford’s expertise in experimental techniques complemented Curie’s deep knowledge of radioactivity, making their partnership a formidable force in the field.
Joint Discoveries
- Identification of New Radioactive Elements:Together, they discovered several new radioactive elements, including polonium and radium. These discoveries laid the foundation for the development of nuclear physics and radiation therapy.
- Nature of Radioactivity:Their collaboration led to a deeper understanding of the nature of radioactivity. They proposed that radioactive decay was a random process, and they developed the concept of half-life to describe the rate of decay.
Significance of Collaboration
Rutherford and Curie’s collaboration not only led to groundbreaking discoveries but also demonstrated Rutherford’s creativity and open-mindedness. He was willing to collaborate with a fellow scientist, regardless of their gender or background, to pursue scientific knowledge.
Their partnership also highlighted the importance of interdisciplinary collaboration in scientific research. By combining their expertise in physics and chemistry, they were able to achieve results that neither could have achieved independently.
Controversy and Criticism

Rutherford’s groundbreaking work was not without its share of controversy and criticism. Initially, he was skeptical of the existence of the atomic nucleus, a concept that would later become central to his model of the atom.
Despite these challenges, Rutherford remained resilient and open-minded. He conducted meticulous experiments to test his hypotheses and was willing to revise his theories in light of new evidence. His ability to think outside the box and to come up with new ideas demonstrates his creativity and resilience.
Impact on Physics
- Rutherford’s work revolutionized our understanding of the atom, leading to the development of the nuclear model.
- His experiments provided the first evidence for the existence of the atomic nucleus, a dense, positively charged region at the center of the atom.
- Rutherford’s work laid the foundation for subsequent discoveries in nuclear physics, including the discovery of isotopes and the development of nuclear energy.
Expert Answers
What was Ernest Rutherford’s most famous experiment?
The gold foil experiment, which led to the discovery of the nuclear model of the atom.
How did Rutherford’s creativity contribute to his scientific discoveries?
Rutherford’s ability to think outside the box and come up with new ideas led to groundbreaking experiments and theories that revolutionized our understanding of the atom.
What was Rutherford’s role in the development of nuclear physics?
Rutherford’s experiments laid the foundation for modern nuclear physics and led to the discovery of the atomic nucleus.